Development and optimisation of a process to biosynthesize reactive iron mineral surfaces for Water treatment purposes
SURFTRAP
From 07/2008 to 06/2011Principal Investigator: Stefan Peiffer
Staff: Susanta Paikaray, Christin Damian
Grant: 03G0714A SURFTRAP
Development and Optimisation of a Process to Biosynthesize Reactive Iron Mineral SURFaces for Water TReAtment Purposes (SURFTRAP).
In this project we aim to develop a low-cost technology to remove ionic constituents from raw waters such as arsenic species. Inexpensive removal of As is certainly one of the major challenges these days in environmental geochemistry, since many remote areas in the developing countries (especially in southeast Asia) with access to water treatment plants are affected by As contamination in drinking water.
The proposed technology is based on the reactivity of schwertmannite, an oxyhydroxo sulfate of the mean stochiometry Fe8O8(OH)6SO4. This mineral typically forms in acidic and sulfate rich mine waters as a secondary mineral upon oxidation of Fe(II) in a biologically mediated process. Schwertmannite can be generated in a biotechnological process after aeration of mining process waters. It forms surface-rich aggregates of needle-like nanocrystals. It rapidly transforms into ferric hydroxides of high specific surface area once exposed to water containing at least some alkalinity. Our rationale follows the concept to make use of this transformation reaction by adding biosynthesized schwertmannite to contaminated raw waters where it generates a large sorption capacity to remove the pollutants. The proposed process to generate reactive surface sites (Fig. 1) is advantageous both, in economical and ecological regard compared to existing techniques. It requires per mol iron oxide formed only 8 % of the amount of alkalinity compared to the use of Fe(III) containing salts (Equation 1), and releases only 4 % of the amount of salts.
FeCl3 + 3H2O --> Fe(OH)3 + 3H+ + 3Cl- (Equation 1)
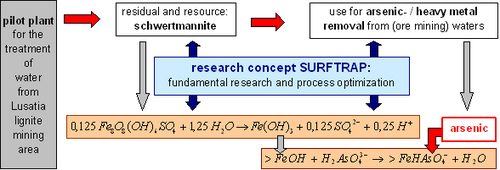
Fig. 1: Scheme of the proposed research concept SURFTRAP.
Schwertmannite can be therefore be used in active water treatment plants instead of FeCl3, (e. g. as pellets). The injection of schwertmannite-sludges in order to generate ‘Reactive Zones’ for In-Situ groundwater remediation can be a further, interesting and innovative application of this residual. The research work deals with -
- Optimisation of the schwertmannite synthesis process with special emphasis on the understanding of the role of biomineralization
- Optimisation of the biotechnological process to generate schwertmannite
- Understanding of the long-term stability of the contaminants bound to the surfaces of the precipitated ferric (hydr)oxides with regard to binding stability and redox state in order to evaluate the potential for disposal of the sequestered substances
- Understanding the kinetic aspects of the interaction between contaminated raw water and the surface of schwertmannite and its transformation products
- Test of a pilot plant to compare the novel technique with conventional treatment technologies with regard to efficiency and costs emphasizing on arsenic containing effluents from mine sites

